MAVERICK™
The Maverick™ Diagnostic System (MDS) uses
silicon chip-based photonic ring resonator
technology to perform multiple simultaneous
rapid tests on a small volume of whole
blood or serum. The system is cloud-connected
for assay protocol retrieval and clinical
oversight.

Maverick™ Immunoassay Analyzer
- Benchtop solution available for On-Site or Core Laboratory testing as well as integrated in Merlin
- Runs four patients simultaneously
- Runs up to 26 tests on a single drop of blood
- 250 patients/day
- High sensitivity and specificity
- Cloud-connected with CLIA/CAP pathology oversight
- Dimensions:
11” high X 16″ wide X 23″ deep

Photonic Ring Resonator Technology
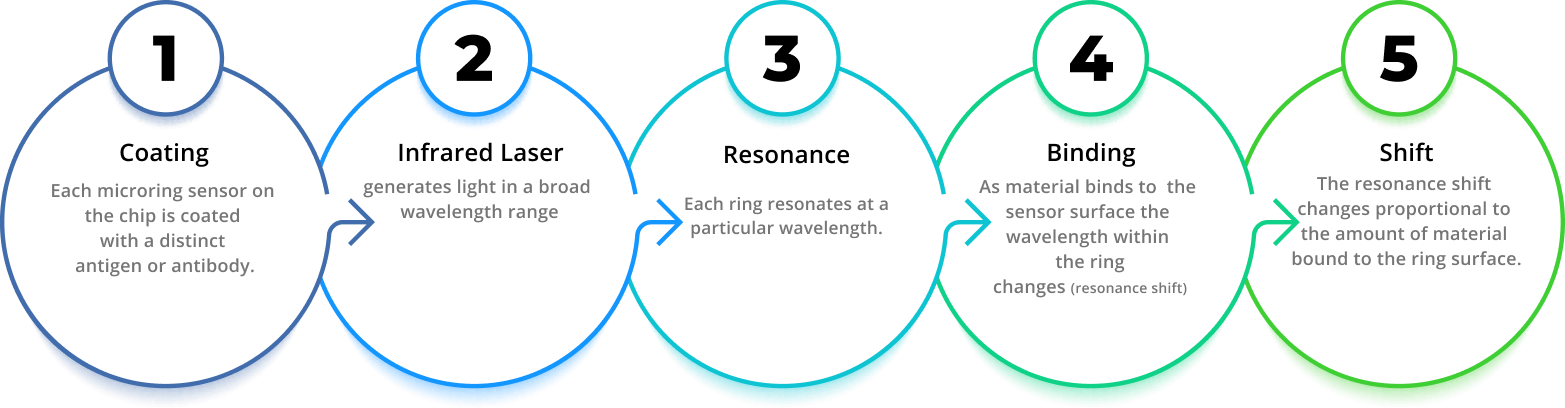
Genalyte has developed multiplex assay detection technology based on silicon photonics that uses ring resonance to measure binding of macromolecules to sensors on a miniature silicon chip. The Maverick™ Diagnostic System detects changes in resonance wavelength as macromolecules, such as autoantibodies, bind to their respective antigens that are bound to the chip; eliminating the need for fluorescent, luminescent, or radioactive labels used in other immunoassay formats.

SARS-CoV-2 Multi-Antigen Serology Panel
Genalyte’s SARS-CoV-2 antibody test measures reactivity levels for 26 IgG and IgM antibody targets with market leading performance.
Tested by Northshore University, Mayo Clinic, and Scripps, Genalyte’s SARS-CoV-2 antibody test had the highest sensitivity and specificity as compared to central lab machines.
How It Works
Watch Maverick™ Technology In Action
31 Patent Issued and 17 Pending
Our proprietary Maverick™ Diagnostic System (MDS) has 31 patents issued and 17 patents pending worldwide.

Improving Overall Patient Care
In order to improve overall patient care with rapid laboratory diagnostics, Genalyte became a clinical diagnostic provider receiving CLIA certification and CAP accreditation.
FDA Clearance and EUA Authorization
The Maverick™ Diagnostic System (MDS) received FDA clearance for RNP (ribonuceloprotein) assay in October 2019.
In addition, the Maverick™ Diagnostic System (MDS) received Emergency Use Authorization (EUA) by the FDA for SARS-CoV-2 Multi-Antigen Serology Panel in October 2020
